Replacement
We use the techniques that do not use living animals and encompass In vitro & In silico (computer based programs) approaches and any other methodologies that eliminate the use of the living animal, e.g., structure–activity relationships.
Refinement
Improvements to scientific procedures and husbandry that minimise actual or potential pain, suffering, distress or lasting harm, and/or improve animal welfare in situations where the use of animals is unavoidable. Refinement also comprises the use of species lower on the phylogenetic scale. Thus, replacing a dog with a mouse is a refinement technique.
Reduction
The concepts of Reduction, decreasing the number of animals necessary in any protocol, is desirable, but the numbers should not be so low that the experiment is compromised, thus to obtain valid statistical compliant results is pivotal while reducing the number of animals in any protocol.
Rehabilitation
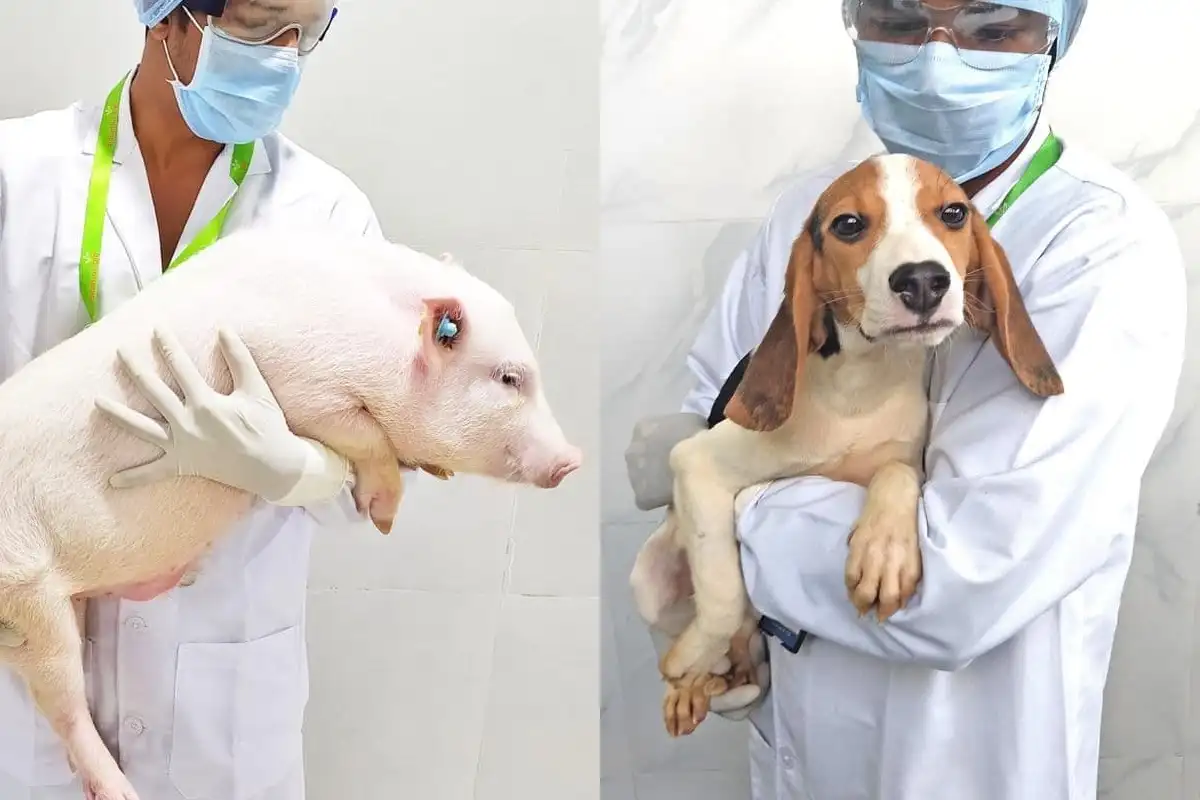
Our scientists have a moral responsibility to the animals after using them in the experiment, we care about the animals after procedure and rehabilitate them after certain period which is also a mandate process as per the legal requirement of CPCSEA.